Life as we don't know it: Some aliens may need sulfuric acid like we need water
"Even very complex organic chemistry is compatible with concentrated sulfuric acid."
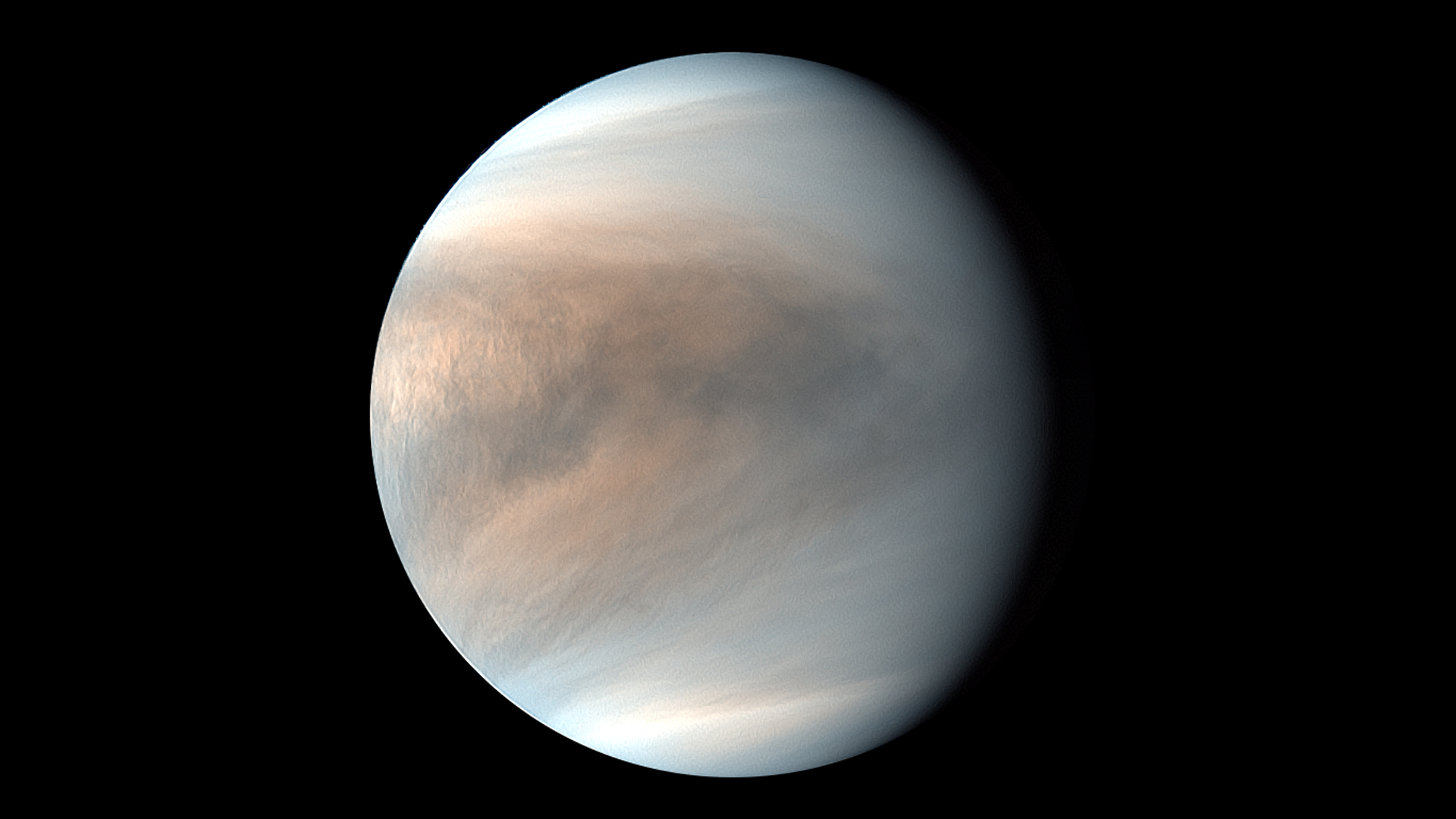
Life as we know it needs water, but life as we don't know it may run on concentrated sulfuric acid.
The chemistry of life as we know it wouldn't work in a place like the huge Saturn moon Titan, where it's so cold that ice behaves more like rock, or in the acidic clouds of Venus. But a different chemistry, which built all the requisite pieces out of different materials, might have a shot. Imagine cells that use methane, sulfuric acid, or even molten rock the way your cells use water.
According to a 2021 study by Massachusetts Institute of Technology (MIT) molecular biologist William Bains and his colleagues, it turns out that, if we're looking for life as we don't know it, the best solvent out there may be concentrated sulfuric acid — the stuff that's floating around in the clouds of Venus.
Sulfuric acid: Hazmat or solvent for life?
At the most basic level, life is just a series of chemical reactions. Those reactions need a medium in which to occur, which chemists call a solvent: something fluid enough that molecules can float around and mingle, and there needs to be a lot of it in one place. But not just any liquid will do; a solvent also needs to help break down and transport the chemicals that cells need to live — without also dissolving important molecules like lipids and amino acids.
Related: Alien life could thrive in Venus' acidic clouds, new study hints
Here on Earth, water fits the bill perfectly, but it may not be the only liquid in the universe capable of supporting the chemistry of life. In a new study, Bains and his colleagues rated several possible solvents based on how they interact with the chemical building blocks of life and how common they should be on rocky planets.
Candidates ranged from the liquid forms of methane and ethane, ammonia and carbon dioxide to even stranger possibilities like pitch and molten rock. The surprising champion is a chemical we Earthlings think of as painfully hostile to life: concentrated sulfuric acid.
Get the Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!
"Even very complex organic chemistry is compatible with concentrated sulfuric acid," MIT astrobiologist Janusz Petkowski, a co-author of the new study, which was published in December in the journal Astrobiology, told Space.com.
A nod to the runners-up
Titan's frigid lakes of methane have been a hotspot for speculation about life as we don't know it ever since NASA's Cassini Saturn orbiter sent home the first photos of them in 2005. Methane and ethane are gases at the temperatures we find here on Earth; to condense into the liquid that fills the dark seas of Titan, they need temperatures around minus 250 degrees Fahrenheit (minus 157 Celsius). But in such extreme cold, atoms and molecules move sluggishly, so chemical reactions happen in slow motion — far too slowly for life to happen, according to Petkowski.
At the other end of the spectrum, molten rock is out because the tremendous heat required to keep rock liquid also breaks down nearly all organic molecules.
Some chemicals, like ammonia, have all the right properties to make good solvents for life's chemistry to happen in. But that may not matter, because the same processes that equip a rocky planet with ammonia are also likely to stock it with water, and when that happens, the two substances almost inevitably mix. That's an interesting prospect for life in its own right, because ammonia lowers the freezing point of water, which opens up a wider range of places where life could survive the cold.
"While ammonia may play a bigger role in an exoplanet biochemistry than it does on Earth," Bains and his colleagues wrote in their recent paper, "It is unlikely to be a solvent in its own right."
Life on an acid world
So, if astrobiologists ever do find alien life with a weird chemistry that uses some liquid other than water, it's not likely to be methane, ammonia, or molten rock — but somewhere out there, alien life just might have cells filled with pure sulfuric acid.
Based on physics models of how solar systems form, sulfuric acid should be fairly common on rocky planets like Venus, and it's definitely good at dissolving things. But surprisingly, some of the most important building blocks for life — things like amino acids and lipids — can float around and do chemistry in pure sulfuric acid just as easily as they can in water.
"Classically, [sulfuric acid] is not seen as a great solvent [for life]," Dirk Schulze-Makuch, an astrobiologist at the Technical University of Berlin who was not involved in the recent study, told Space.com. "However, Bains and co-authors showed that an amazingly large number and various types of organics are stable in concentrated sulfuric acid. That was quite surprising."
Petkowski participated in some recent experiments that found that some peptides (short chains of amino acids) were actually stable in concentrated sulfuric acid for months on end — so stable that eventually the researchers just got bored with the measurements, according to Petkowski. Earlier experiments found that 19 out of the 20 amino acids that form proteins in the human body don't dissolve in concentrated sulfuric acid. And a study that's still awaiting peer review found that not only can lipids (the molecules that make up our cell membranes) withstand concentrated sulfuric acid, they actually start to form tiny fluid-filled sacs that look a lot like vesicles, the precursors of membranes.
How does that happen? Strangely, the key is water. Sulfuric acid on Earth is usually found mixed with water, not in its pure form; it's really water with some acid in it. And in that mixture, sulfuric acid catalyzes chemical reactions between water and the bonds that hold peptides together. Take away either the water or the acid, and those reactions can't happen — so peptides are stable in either solvent, but not in a mixture of the two.
"Concentrated sulfuric acid is a very different substance from diluted acid," said Petkowski. "It is a common misconception that sulfuric acid destroys all organic chemistry. This is wrong. It is a very aggressive solvent, but it is very aggressive to specific parts in organic molecules, like sugars." (For all its mildness to amino acids and lipids, sulfuric acid will absolutely destroy sugars, which make up a lot of the scaffolding on which life is built.)
Somewhere in the universe, sulfuric acid lakes could be teeming with life forms whose basic chemistry is only a little different from ours.
Related: The search for alien life
What are alien cells made of?
Alien life forms that use sulfuric acid in place of water would probably look surprisingly similar to Earth life, with some subtle but important differences in their chemical makeup.
"We should understand sulfuric acid as an environment that is not so alien as we think it is," said Petkowski. (That doesn't mean you can safely swim in concentrated sulfuric acid; your cells did not evolve for that. Please do not test it.)
For example, life in a sulfuric acid sea would have to use something sturdier in place of sugars, which disintegrate very quickly in sulfuric acid. On Earth, sugars are how cells store energy, and they also make up parts of cell walls. Aliens on Venus would use some other type of molecule to do the same things without falling apart.
"You have to adapt your organic chemistry to the solvent," said Petkowski. "You have to choose the chemical LEGO building blocks properly, but you can arrive with functionally the same structures at the end."
Other differences might be more subtle. Concentrated sulfuric acid doesn't break down most of the amino acids our bodies use, but it does cause small changes in their "side chains," strings of atoms that stick out from the molecule like a tail. And just a single chemical bond makes the difference between a peptide that's stable in sulfuric acid and one that breaks down. So aliens on an acidic world could still have cells made of amino acids and proteins, but they might look just a tiny bit different than ours.
The membranes that surround cells — keeping the chemical mechanisms of life inside and everything else outside — may look strikingly familiar, though. Those vesicle-like globules that appeared when Petkowski and his colleagues put lipids into sulfuric acid actually formed in exactly the same way that vesicles form in water: a process called pearling. First, the lipids form tubes, and then those tubes break apart into little vesicles. Life on an acidic world might use a different set of lipids than the ones in our cell membranes, but the physics of membrane formation would look the same, if Petkowski and his colleagues are right.
Astrobiologists like Petkowski, Schulze-Makuch, and others still aren't exactly sure how all the pieces of life as we don't know it might come together. But, as Petkowski pointed out, they're also not sure how a few groups of molecules here on Earth made the leap from chemistry to life.
"We are as far away from figuring out [the] origin of life in water as we are in concentrated sulfuric acid," Petkowski said.
Join our Space Forums to keep talking space on the latest missions, night sky and more! And if you have a news tip, correction or comment, let us know at: community@space.com.

Kiona Smith is a science writer based in the Midwest, where they write about space and archaeology. They've written for Inverse, Ars Technica, Forbes and authored the book, Peeing and Pooping in Space: A 100% Factual Illustrated History. They attended Texas A&M University and have a degree in anthropology.